
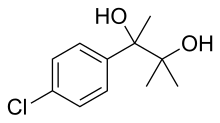
Phenaglycodol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.124 |
| Chemical and physical data | |
| Formula | C11H15ClO2 |
| Molar mass | 214.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phenaglycodol (brand names Acalmid, Acalo, Alterton, Atadiol, Felixyn, Neotran, Pausital, Remin, Sedapsin, Sinforil, Stesil, Ultran) is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties. It is related pharmacologically to meprobamate, though it is not a carbamate.
Synthesis
p-Chloroacetophenone and NaCN are reacted together to give the corresponding cyanohydrin (cf Strecker synthesis), CID:12439573. The cyano group is then hydrated in acid to the corresponding amide, p-chloroatrolactamide, CID:15255544 (4). The amide group is then further hydrolyzed with a 2nd equivalent of water in concentrated lye to p-chloroatrolactic acid, [4445-13-0] (5). Esterification to Ethyl p-chloroatrolactate [100126-96-3](6). Finally, nucleophilic addition a couple of equivalents of MeMgI are added to the ester give Phenaglycodol (7) crystals.
A mixed Pinacol coupling rxn between 4-chloroacetophenone [99-91-2] and acetone with magnesium activated with a small amount of trimethylsilyl chloride gave a 40% yield of phenglycodol.
- See "Novel trifluoromethyl derivatives of substituted diols" U.S. Patent 3,134,819 also.
- A Pinacol rearrangement occurs in acidic water:
See also
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
|
Gabapentinoids |
|
| Antidepressants |
|
|
Sympatholytics |
|
| Others | |
| |

