
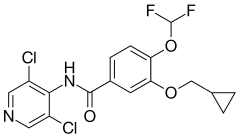
Roflumilast
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daxas, Daliresp, Zoryve, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 79% |
| Protein binding | 99% |
| Metabolism | Hepatic via CYP1A2 & CYP3A4 |
| Elimination half-life | 17 hours (30 hours [active metabolite]) |
| Excretion | Urine (70%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.960 |
| Chemical and physical data | |
| Formula | C17H14Cl2F2N2O3 |
| Molar mass | 403.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Roflumilast, sold under the trade name Daxas among others, is a drug that acts as a selective, long-acting inhibitor of the enzyme phosphodiesterase-4 (PDE-4). It has anti-inflammatory effects and is used as an orally administered drug for the treatment of inflammatory conditions of the lungs such as chronic obstructive pulmonary disease (COPD).
In June 2010, it was approved in the European Union for severe COPD associated with chronic bronchitis. In February 2011, it gained FDA approval in the United States for reducing COPD exacerbations. It is available as a generic medication.
Medical uses
Roflumilast is indicated for the treatment of severe chronic obstructive pulmonary disease (COPD) and for the treatment of plaque psoriasis.
It is used in the prevention of exacerbations (lung attacks) in severe chronic obstructive pulmonary disease (COPD).
Adverse effects
Common (1–10% incidence) adverse effects include:
- Diarrhea
- Weight loss
- Nausea
- Headache
- Insomnia
- Decreased appetite
- Abdominal pain
- Rhinitis
- Sinusitis
- Urinary tract infection
- Depression
External links
- "Roflumilast". Drug Information Portal. U.S. National Library of Medicine.
| PDE1 | |
|---|---|
| PDE2 | |
| PDE3 | |
| PDE4 |
|
| PDE5 | |
| PDE7 | |
| PDE9 | |
| PDE10 | |
| PDE11 | |
| Non-selective | |
| Unsorted | |
See also: Receptor/signaling modulators | |
| Products |
|
|---|---|
| Predecessors and acquired companies |
|
| People | |