Trimethoprim/sulfamethoxazole

Trimethoprim (top) and sulfamethoxazole (bottom)
| |
| Combination of | |
|---|---|
| Sulfamethoxazole | Sulfonamide antibiotic |
| Trimethoprim | Dihydrofolate reductase inhibitor |
| Clinical data | |
| Trade names | Bactrim, Cotrim, Septra, others |
| Other names | TMP/SMX, Co-trimoxazole (BAN UK) |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| (verify) | |
Trimethoprim/sulfamethoxazole, (also known as Cotrimoxazole) sold under the brand name Bactrim among others, is a fixed-dose combination antibiotic medication used to treat a variety of bacterial infections. It consists of one part trimethoprim to five parts sulfamethoxazole. It is used to treat urinary tract infections, methicillin-resistant Staphylococcus aureus (MRSA) skin infections, travelers' diarrhea, respiratory tract infections, and cholera, among others. It is used both to treat and prevent pneumocystis pneumonia and toxoplasmosis in people with HIV/AIDS and other causes of immunosuppression. It can be given by mouth or intravenously.
Trimethoprim/sulfamethoxazole is on the World Health Organization's List of Essential Medicines and is also available as a generic medication. In 2020, it was the 121st most commonly prescribed medication in the United States, with more than 5 million prescriptions.
Medical uses
Pneumocystis jirovecii pneumonia
Trimethoprim/sulfamethoxazole (TMP/SMX) is the medicine most commonly used to prevent Pneumocystis jirovecii pneumonia (PCP) People who get Pneumocystis pneumonia have a medical condition that weakens their immune system, like HIV/AIDS, or take medicines (such as corticosteroids) that reduce the body's ability to fight bacterial and viral infections. People with HIV/AIDS are less likely to get Pneumocystis pneumonia as a result of antiretroviral therapy (ART). However, Pneumocystis pneumonia is still a substantial public health problem. Most of what is scientifically known about Pneumocystis pneumonia and its treatment comes from studying people with HIV/AIDS.
Susceptibility
Organisms against which trimethoprim/sulfamethoxazole can be effective include:
- Acinetobacter spp.
- Aeromonas spp.
- Alcaligenes/Achromobacter spp.
- Bartonella henselae
- Bordetella pertussis (pertussis)
- Brucella spp.
- Burkholderia cepacia
- Burkholderia mallei (glanders)
- Burkholderia pseudomallei (melioidosis)
- Chlamydia spp.
- Chryseobacterium meningosepticum
- Citrobacter spp.
- Enterobacter spp.
- Enterococcus spp.
- Escherichia coli
- Haemophilus spp.
- Hafnia alvei
- Kingella spp.
- Klebsiella granulomatis
- Klebsiella pneumoniae
- Legionella spp.
- Listeria monocytogenes (listeriosis)
- Moraxella catarrhalis
- Morganella morganii
- Mycobacterium tuberculosis (tuberculosis)
- Neisseria gonorrhoeae (gonorrhoea)
- Neisseria meningitidis (meningococcal disease)
- Nocardia spp.
- Plesiomonas shigelloides
- Pneumocystis jirovecii
- Proteus mirabilis
- Proteus vulgaris
- Providencia rettgeri
- Providencia stuartii
- Salmonella typhi (typhoid fever)
- Non-typhi (food poisoning) Salmonella
- Serratia spp.
- Shigella spp.
- Staphylococcus aureus
- Staphylococcus epidermidis
- Staphylococcus saprophyticus
- Stenotrophomonas maltophilia
- Streptococcus agalactiae
- Streptococcus pneumoniae
- Streptococcus pyogenes
- Streptococcus viridans
- Toxoplasma gondii (toxoplasmosis)
- Tropheryma whippelii (Whipple's disease)
- Vibrio cholerae (cholera)
- Yersinia enterocolitica
- Yersinia pestis (bubonic plague)
- Yersinia pseudotuberculosis
The only notable nonsusceptible organisms are Pseudomonas aeruginosa, the mycoplasmae and Francisella tularensis (the causative organism of tularaemia).
Pregnancy and breast feeding
Its use during pregnancy is contraindicated, although it has been placed in Australian pregnancy category C. Its use during the first trimester (during organogenesis) and 12 weeks prior to pregnancy has been associated with an increased risk of congenital malformations, especially malformations associated with maternal folic acid deficiency (which is most likely related to the mechanism of action of co-trimoxazole) such as neural tube defects such as spina bifida, cardiovascular malformations (e.g. Ebstein's anomaly), urinary tract defects, oral clefts, and club foot in epidemiological studies. Its use later on during pregnancy also increases the risk of preterm labour (odds ratio: 1.51) and low birth weight (odds ratio: 1.67). Animal studies have yielded similarly discouraging results.
It appears to be safe for use during breastfeeding as long as the baby is healthy.
Babies
Its use in those less than 2 months of age is not recommended due to the risk of adverse side effects.
Adverse effects
Common side effects include nausea, vomiting, rash, and diarrhea. Severe allergic reactions and Clostridium difficile infection may occasionally occur. Its use in pregnancy is not recommended. It appears to be safe for use during breastfeeding as long as the baby is healthy. Trimethoprim/sulfamethoxazole generally results in bacterial death. It works by blocking the making and use of folate by the microorganisms.
Contraindications
Contraindications include the following:
- Known hypersensitivity to trimethoprim, sulphonamides or any other ingredients in the formulations
- Pregnancy
- Severe hepatic failure, marked liver parenchymal damage or jaundice.
- Serious haematological disorders and porphyria (due to the sulfonamide component of the preparation).
- Severe chronic kidney disease (CrCl <15 ml/min) where repeated measurements of the plasma concentration cannot be performed
- Co-trimoxazole should not be given to neonates during the first 6 weeks, except for the treatment/prophylaxis of Pneumocystis jirovecii (P. carinii) in infants of four weeks of age or greater.
Interactions
Its use is advised against in people being concomitantly treated with:
- ACE inhibitors like captopril, enalapril, lisinopril, perindopril, and ramipril due to the potential for additive hyperkalaemic effects
- Prilocaine — additive risk of methaemoglobinaemia
- Antiarrhythmics like amiodarone (increased risk of ventricular arrhythmias) and dofetilide (increased risk of QT interval prolongation)
- Antibacterials like dapsone (increases plasma levels of both drugs), methenamine (increased risk of crystalluria) and rifampicin (as it may lead to an increased plasma level of rifampicin and lower plasma levels of trimethoprim)
- Anticoagulants like warfarin and acenocoumarol — anticoagulant effects of either drug is potentiated by this combination
- Sulfonylureas — effects enhanced
- Phenytoin, half-life of phenytoin is increased
- Antifolates like pyrimethamine, proguanil and methotrexate increase the risk of associated side effects like bone marrow toxicity, folic acid supplementation should be considered. A significant risk of megaloblastic anaemia exists with doses of pyrimethamine in excess of 25 mg/wk.
- Antivirals, more specifically, lamivudine (increased plasma concentrations of lamivudine), zalcitabine (increased plasma concentrations of zalcitabine) and zidovudine (increased risk of haematological reactions)
- Procainamide and/or amantadine may have their plasma concentrations increased bilaterally or unilaterally.
- Clozapine and other antipsychotics — increased risk of haematological side effects
- Nucleoside analogue antineoplastics like azathioprine and mercaptopurine — increased risk of haematological toxicity
- Digoxin — increase in digoxin levels in a proportion of elderly patients
- Diuretics — elderly patients receiving thiazide diuretics are at a heightened risk for developing thrombocytopaenia while on co-trimoxazole
- Ciclosporin — patients who have received a kidney transplant and are receiving co-trimoxazole and ciclosporin concomitantly are at an increased risk of having a reversible deterioration in their kidney function.
- Spironolactone — concurrent use can increase the likelihood of hyperkalemia, especially in the elderly. The trimethoprim portion acts to prevent potassium excretion in the distal tubule of the nephron.
- Potassium aminobenzoate — effects of sulfonamides (like sulfamethoxazole) inhibited.
- Laboratory tests — trimethoprim and sulfonamides have been reported to interfere with diagnostic tests, including serum-methotrexate and elevated serum creatinine levels, also urea, urinary glucose and urobilinogen tests.
Overdose
Likely signs of toxicity include:
- Nausea
- Vomiting
- Dizziness
- Headache
- Mental depression
- Confusion
- Thrombocytopenia
- Uremia
- Bone marrow depression
- Loss of appetite
- Colic
- Drowsiness
- Unconsciousness
The recommended treatment for overdose includes:
- Administration of activated charcoal
- Stomach pumping
- General supportive measures
- Haemodialysis, which is moderately effective in clearing co-trimoxazole from the plasma.
- Calcium folinate treatment in cases of blood dyscrasias
- Forcing oral fluids
Alkalinisation of the urine may reduce the toxicity of sulfamethoxazole, but it may increase the toxic effects of trimethoprim.
Pharmacology
The synergy between trimethoprim and sulfamethoxazole was first described in the late 1960s. Trimethoprim and sulfamethoxazole have a greater effect when given together than when given separately, because they inhibit successive steps in the folate synthesis pathway. They are given in a one-to-five ratio in their tablet formulations so that when they enter the body their concentration in the blood and tissues is roughly one-to-twenty — the exact ratio required for a peak synergistic effect between the two.
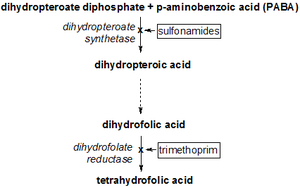
Sulfamethoxazole, a sulfonamide, induces its therapeutic effects by interfering with the de novo (that is, from within the cell) synthesis of folate inside microbial organisms such as protozoa, fungi and bacteria. It does this by competing with p-aminobenzoic acid (PABA) in the biosynthesis of dihydrofolate.
Trimethoprim serves as a competitive inhibitor of dihydrofolate reductase (DHFR), hence inhibiting the de novo synthesis of tetrahydrofolate, the biologically active form of folate.
Tetrahydrofolate is crucial in the synthesis of purines, thymidine, and methionine which are needed for the production of DNA and proteins during bacterial replication. Thus the net effect of each of these drugs is a bacteriostatic halt in replication. When combined, TMP and SMX are bactericidal.
The effects of trimethoprim causes a backlog of dihydrofolate (DHF) and this backlog can work against the inhibitory effect the drug has on tetrahydrofolate biosynthesis. This is where the sulfamethoxazole comes in; its role is in depleting the excess DHF by preventing it from being synthesised in the first place.
Co-trimoxazole was claimed to be more effective than either of its components individually in treating bacterial infections, although this was later disputed. Because it has a higher incidence of adverse effects, including allergic responses, its use has been restricted in many countries to very specific circumstances where its improved efficacy has been demonstrated. It may be effective in a variety of upper and lower respiratory tract infections, kidney and urinary tract infections, gastrointestinal tract infections, skin and wound infections, sepsis, and other infections caused by sensitive organisms. Co-trimoxazole decreases the risk of recurrence of retinochoroiditis. The global problem of advancing antimicrobial resistance has led to a renewed interest in the use of co-trimoxazole more recently.
| Component | Tmax (h) | Vd (L) | Protein binding | t1/2 (h) | Excretion |
|---|---|---|---|---|---|
| Sulfamethoxazole | 1-4 | 20 | 66% | 8-10 | Renal |
| Trimethoprim | 1-4 | 130 | 42-45% | 10 | Renal |
Society and culture
Legal status
| Indication |
FDA-labelled indication? |
TGA-labelled indication? |
MHRA-labelled indication? |
Literature support |
|---|---|---|---|---|
| Acute infective exacerbation of COPD | Yes | No | No | Clinical trials are lacking. |
| Prophylaxis in HIV-infected individuals | No | No | No | Effective in one Ugandan study on morbidity, mortality, CD4-cell count, and viral load in HIV infection. |
| Otitis media | Paediatric population only | No | Yes | Clinical trials have confirmed its efficacy in chronic active otitis media and acute otitis media. |
| Travellers' diarrhoea, treatment & prophylaxis | Yes | No | No | Clinical trials have confirmed its efficacy as a treatment for travellers' diarrhoea. |
| Urinary tract infection | Yes | No | Yes | Clinical trials have confirmed its efficacy in this indication. |
| Bacterial infections | ||||
| Acne vulgaris | No | No | No | At least one clinical trial supports its use in this indication. |
| Listeria | No | Yes | No | Well-designed clinical trials are lacking. |
| Melioidosis | No | Yes | No | Clinical trials have confirmed its efficacy, with or without adjunctive doxycycline; although, co-trimoxazole alone seems preferable. |
| Pertussis (whooping cough) | No | No | No | One cochrane review supports its efficacy in preventing the spread of pertussis. |
| Shigellosis | Yes | Yes | No | Generally accepted treatment for shigellosis. A recent Cochrane review found that while it is an effective treatment for shigellosis it also produces more significant adverse effects than other antibiotic drugs. |
| Staphylococcus aureus infections | No | No | No | In vitro and in vivo activity against both non-resistant and methicillin-resistant Staphylococcus aureus (MRSA) infections. |
| Tuberculosis | No | No | No | In vitro and in vivo activity against both nonresistant and MDR strains of TB. |
| Whipple's disease | No | No | No | Co-trimoxazole is the recommended standard treatment for whipple's disease in some treatment protocols. |
| Fungal and protozoal infections | ||||
| Isosporiasis | No | No | No | Clinical trials have confirmed its use in this indication. |
| Malaria | No | No | No | Clinical trials have confirmed its efficacy in both the treatment and prevention of malaria. |
| Pneumocystis jirovecii pneumonia | Yes | Yes | Yes | Its use as a prophylactic treatment is supported by one clinical trial involving children with acute lymphoblastic leukaemia. Other than this and one other clinical trial into its efficacy as a treatment for pneumocystis pneumonia, data on its use in both the treatment and prevention of pneumocystis pneumonia is significantly lacking. |
| Toxoplasmosis | Yes | Prevention only | Yes | Clinical trials have confirmed its prophylactic and therapeutic utility in cases of toxoplasmosis. |
Trade names
Trimethoprim/sulfamethoxazole may be abbreviated as SXT, SMZ-TMP, TMP-SMX, TMP-SMZ, or TMP-sulfa.
Co-trimoxazole (British Approved Name (BAN)) is manufactured and sold by many different companies. The following list of brand names is incomplete:
- Bactrim, Bactrimel (manufactured by Roche and distributed in Europe)
- Bactrom (Venezuela)
- Bibactin (manufactured by PPM and distributed in Cambodia and some African countries)
- Biseptol
- Sumetrolim
- Co-trimoxazole (Sandoz)
- Cotrim
- Deprim (AFT Pharmaceuticals)
- Diseptyl (Israel)
- Graprima Forte Kaplet (manufactured by PT Graha Farma and distributed in Indonesia)
- Infectrin, Bactrim (Brazil)
- Novo-Trimel
- Primotren (Lek in Slovenia and other countries)
- Resprim
- Sanprima (manufactured by PT Sanbe Farma and distributed in Indonesia)
- Septra (Aspen Pharmacare and formerly GlaxoSmithKline)
- Septram (Panama)
- Septran (GlaxoSmithKline)
- Septrin (Spain)
- Sulfatrim
- Teva-Trimel
- Trisul
- Vactrim (manufactured and distributed in Laos)
Economics
Trimethoprim/sulfamethoxazole is relatively inexpensive as of 2019.
External links
- "Sulfamethoxazole mixture with trimethoprim". Drug Information Portal. U.S. National Library of Medicine.
|
Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
|
||||||||||||||||
|
Anaerobic DNA inhibitors |
|
||||||||||||||||
| RNA synthesis |
|
||||||||||||||||
| |||||||||||||||||
| Alveo- late |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hetero- kont |
|||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||