
Apronal
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.677 |
| Chemical and physical data | |
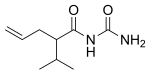
| Formula | C9H16N2O2 |
| Molar mass | 184.239 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Apronal (brand name Sedormid), or apronalide, also known as allylisopropylacetylurea or allylisopropylacetylcarbamide, is a hypnotic/sedative drug of the ureide (acylurea) group synthesized in 1926 by Hoffmann-La Roche that is no longer used except in Japan (See Japanese article). Though it is not a barbiturate, apronalide is similar in structure to the barbiturates (being an open-chain carbamide instead of having a heterocyclic ring). In accordance, it is similar in action to the barbiturates, although considerably milder in comparison (formerly used as a daytime sedative at doses of 1 to 2 grams every 3 to 4 hours). Upon the finding that it caused patients to develop thrombocytopenic purpura, apronalide was withdrawn from clinical use.
See also
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|