
Atevirdine
| |
| Names | |
|---|---|
|
Preferred IUPAC name
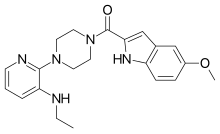
{4-[3-(Ethylamino)pyridin-2-yl]piperazin-1-yl}(5-methoxy-1H-indol-2-yl)methanone | |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H25N5O2 | |
| Molar mass | 379.46 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Atevirdine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.
Synthesis

Preparation of the pyridylpiperazine moiety starts by aromatic displacement of chlorine from 2-chloro-3-nitropyridine by piperazine to give 3. The secondary amine is then protected as its BOC derivative by reaction with di-tert-butyl dicarbonate (Boc anhydride) to give 4. The nitro group is then reduced by catalytic hydrogenation. Reductive alkylation with acetaldehyde in the presence of lithium cyanoborohydride gives the corresponding N-ethyl derivative. The protecting group is then removed by reaction with TFA. Reaction of the resulting amine with the imidazolide derivative of 5-methoxy-3-indoleacetic acid produces the amide reverse transcriptase inhibitor, atevirdine.
See also
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
|
Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
|
Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |