
Avamys
 | |
| Clinical data | |
|---|---|
| Trade names | Flonase Sensimist, Veramyst, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Intranasal, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91% |
| Metabolism | Intranasal Liver (CYP3A4-mediated) |
| Elimination half-life | 15 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.130 |
| Chemical and physical data | |
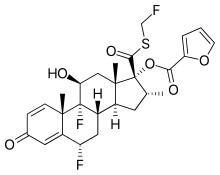
| Formula | C27H29F3O6S |
| Molar mass | 538.58 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fluticasone furoate, sold under the brand name Flonase Sensimist and formerlyVeramyst, among others, is a corticosteroid for the treatment of non-allergic and allergic rhinitis administered by a nasal spray. It is also available as an inhaled corticosteroid to help prevent and control symptoms of asthma. It is derived from cortisol. Unlike fluticasone propionate, which is only approved for children four years and older, fluticasone furoate is approved in children as young as two years of age when used for allergies.
It was approved for medical use in the United States in April 2007, and in the European Union in November 2008. In 2020, fluticasone was the 23rd most commonly prescribed medication in the United States, with more than 24 million prescriptions.
Society and culture
Brand names
In the US it is marketed by GlaxoSmithKline for asthma as Arnuity Ellipta and is only available with a prescription. It is marketed over-the-counter for allergic rhinitis as Flonase Sensimist. The Veramyst brand name has been discontinued in the US. It is also marketed as Allermist (Japan, アラミスト) and Avamys (Australia, Canada, EU, South Africa, South America, Mexico, Israel, Italy, India, Taiwan and South Korea).
The combination drugs fluticasone furoate/umeclidinium bromide/vilanterol, marketed as Trelegy Ellipta, and fluticasone furoate/vilanterol, marketed as Breo Ellipta (US, Canada, New Zealand) and Relvar Ellipta (EU, UK), are approved for use in the United States for long-term maintenance treatment of airflow obstruction in people with chronic obstructive pulmonary disease (COPD). They are also approved for the treatment of asthma.
See also
External links
- "Fluticasone furoate". Drug Information Portal. U.S. National Library of Medicine.
| Glucocorticoids |
|
||||
|---|---|---|---|---|---|
| Antiglucocorticoids |
|
||||
| Synthesis modifiers | |||||
| |||||
|
Decongestants and other nasal preparations (R01)
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Topical |
|
||||||||||
| Systemic use: Sympathomimetics |
|||||||||||
| |||||||||||
| Subsidiaries |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predecessors, acquisitions |
|||||||||
| Products |
|
||||||||
| People |
|
||||||||
| Litigation | |||||||||
| Other | |||||||||