Ethinamate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.355 |
| Chemical and physical data | |
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Ethinamate (Valamin, Valmid) is a short-acting carbamate-derivative sedative-hypnotic medication used to treat insomnia. Regular use leads to drug tolerance, and it is usually not effective for more than 7 days. Prolonged use can lead to dependency.
Ethinamate has been replaced by other medicines (particularly benzodiazepines), and it is not available in the Netherlands, the United States or Canada.
It is a schedule IV substance in the United States.
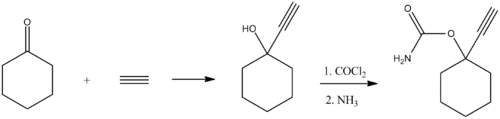
Synthesis
Ethinamate (1-ethynylcyclohexanone carbamate) is synthesized by combining acetylene with cyclohexanone to make 1-ethynylcyclohexanol, and then transforming this into a carbamate by the subsequent reaction with phosgene, and later with ammonia. Some lithium metal or similar is used to make the acetylene react with the cyclohexanone in the first step.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|