Monastrol
Подписчиков: 0, рейтинг: 0
| |

| |
| Names | |
|---|---|
|
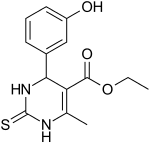
IUPAC name
ethyl 4-(3-hydroxyphenyl)-6-methyl-2-sulfanylidene-3,4-dihydro-1H-pyrimidine-5-carboxylate
| |
| Other names
Monastrol
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
|
PubChem CID
|
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H16N2O3S | |
| Molar mass | 292.35344 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
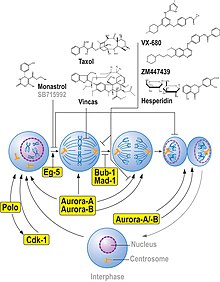
Monastrol is a cell-permeable small molecule inhibitor discovered by Thomas U. Mayer in the lab of Tim Mitchison. Monastrol was shown to inhibit the kinesin-5 (also known as KIF11, Kinesin Eg5), a motor protein important for spindle bipolarity.
Mechanism of action
Monastrol binds to a long loop that is specific to the Eg5 (also known as KIF11 or kinesin-5) kinesin family, and allosterically inhibits ATPase activity of the kinesin
|
Receptor (ligands) |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Transporter (blockers) |
|
||||||||||
|
Enzyme (inhibitors) |
|
||||||||||