
Oxaprotiline
 | |
| Clinical data | |
|---|---|
| Routes of administration |
Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
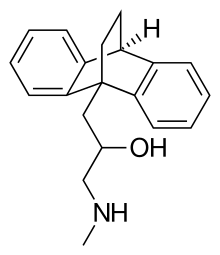
| Formula | C20H23NO |
| Molar mass | 293.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Oxaprotiline (developmental code name C 49-802 BDA), also known as hydroxymaprotiline, is a norepinephrine reuptake inhibitor belonging to the tetracyclic antidepressant (TeCA) family and is related to maprotiline. Though investigated as an antidepressant, it was never marketed.
Pharmacology
Dextroprotiline acts as a potent norepinephrine reuptake inhibitor and H1 receptor antagonist, as well as a very weak α1-adrenergic receptor antagonist. It has negligible affinity for the serotonin transporter,dopamine transporter, α2-adrenergic receptor, and muscarinic acetylcholine receptors. Whether it has any antagonistic effects on the 5-HT2, 5-HT7, or D2 receptors like its relative maprotiline is unclear.
Levoprotiline acts as a selective H1 receptor antagonist, with no affinity for adrenaline, dopamine, muscarinic acetylcholine, or serotonin receptors, or any of the monoamine transporters.
Chemistry
Oxaprotiline is a racemic compound composed of two isomers, R(−)- or levo- oxaprotiline (levoprotiline; CGP-12,103-A), and S(+)- or dextro- oxaprotiline (dextroprotiline; CGP-12,104-A). Both enantiomers are active, with the levo- form acting as an antihistamine and the dextro- form having an additional pharmacology (see above), but with both unexpectedly still retaining antidepressant effects.
See also
| |||||||||||||||||||||
| |||||||||||||||||||||
|
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Classes | |
|---|---|
|
Antidepressants (Tricyclic antidepressants ) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|