- American Parkinson Disease Association
- Animal models of Parkinson's disease
- Biotie Therapies
- Braak staging
- Bradyphrenia
- Causes of Parkinson's disease
- Cloudy Times
- Contursi Terme
- DaT scan
- Davis Phinney Foundation
- Diane G. Cook
- Dietary management of Parkinson's disease
- Dyskinesia
- Evangeline Lodge Land Lindbergh
- Fox Trial Finder
- Gene therapy in Parkinson's disease
- Geraets-Smits v Stichting Ziekenfonds
- Gut–brain axis
- History of Parkinson's disease
- Hoehn and Yahr scale
- Ioflupane (123I)
- Kinesia paradoxa
- Lee Silverman voice treatment
- Leo and Me
- Levodopa-induced dyskinesia
- Lineage Performing Arts Center
- Management of Parkinson's disease
- Micrographia (handwriting)
- MultiPark
- Pacific Northwest Udall Center
- Palilalia
- Parkinson's Association
- Parkinson's Disease (journal)
- Parkinson's Europe
- Parkinson's Foundation
- Parkinson-plus syndrome
- Parkinsonism
- Parkinson Association of the Rockies
- Pathophysiology of Parkinson's disease
- PD-0298029
- Rapid eye movement sleep behaviour disorder and Parkinson's disease
- Shake it Up Australia Foundation
- Signs and symptoms of Parkinson's disease
- Thalamotomy
- The Michael J. Fox Foundation
- Transcranial direct stimulation in Parkinson's disease gait rehabilitation
- Tremorine
- Unified Parkinson's disease rating scale
- Washington State Parkinson Disease Registry
- World Parkinson Congress
- Management of Parkinson's disease
- People with Parkinson's disease
Parkinson's disease
| Parkinson's disease | |
|---|---|
| Other names | Parkinson disease, idiopathic or primary parkinsonism, hypokinetic rigid syndrome, paralysis agitans, shaking palsy |
 | |
| Illustration of Parkinson's disease by William Richard Gowers, first published in A Manual of Diseases of the Nervous System (1886), and based on 1879 photographs attributed to Albert Londe | |
| Specialty | Neurology |
| Symptoms | Tremor, rigidity, slowness of movement, difficulty walking |
| Complications | Dementia, depression, anxiety, eating problems, and sleep problems |
| Usual onset | Age over 60 |
| Causes | Unknown |
| Risk factors | Pesticide exposure, head injuries |
| Diagnostic method | Based on symptoms |
| Differential diagnosis | Dementia with Lewy bodies, progressive supranuclear palsy, essential tremor, antipsychotic use |
| Treatment | Medications, surgery |
| Medication | L-DOPA, dopamine agonists |
| Prognosis | Life expectancy about 7–15 years |
| Frequency | 6.2 million (2015) |
| Deaths | 117,400 (2015) |
| Named after | James Parkinson |
Parkinson's disease (PD), or simply Parkinson's, is a chronic degenerative disorder of the central nervous system that mainly affects the motor system. The symptoms usually emerge slowly, and as the disease worsens, non-motor symptoms become more common. Early symptoms are tremor, rigidity, slowness of movement, and difficulty with walking. Problems may also arise with cognition, behaviour, sleep, and sensory systems.Parkinson's disease dementia becomes common in advanced stages of the disease.
The motor symptoms of the disease result from the death of nerve cells in the substantia nigra, a region of the midbrain that supplies dopamine to the basal ganglia. The cause of this cell death is poorly understood, but involves the aggregation of the protein alpha-synuclein into Lewy bodies within the neurons. Collectively, the main motor symptoms are known as parkinsonism or a parkinsonian syndrome.
The cause of PD is unknown, but a combination of genetic and environmental factors are believed to play a role. Those with an affected family member are at an increased risk of getting the disease, with certain genes known to be inheritable risk factors. Environmental risks include exposure to pesticides, and prior head injuries; a history of exposure to trichloroethylene is also suspected. Conversely, caffeine and nicotine appear to be protective.
Diagnosis is mainly based on symptoms, with motor symptoms being the most frequently presented. Tests such as neuroimaging (magnetic resonance imaging or imaging to look at dopamine neuronal dysfunction known as DaT scan) are used to help rule out other diseases. Parkinson's disease typically occurs in people over the age of 60, of whom about one percent are affected. Males are affected at a ratio of around 3:2 compared with females. When it is seen in people before the age of 50, it is called early-onset PD. By 2015, PD affected 6.2 million people and resulted in about 117,400 deaths globally. The number of people with PD older than fifty is expected to double by 2030. The average life expectancy following diagnosis is 7–15 years.
No cure for PD is known; treatment aims to reduce the effects of the symptoms.Initial treatment is typically with the medications levodopa (L-DOPA), MAO-B inhibitors, or dopamine agonists. As the disease progresses, these medications become less effective, while at the same time producing a side effect marked by involuntary muscle movements. At that time, medications may be used in combination and doses may be increased. Diet and certain forms of rehabilitation have shown some effectiveness at improving symptoms.Surgery to place microelectrodes for deep brain stimulation has been used to reduce severe motor symptoms where drugs are ineffective. Evidence for treatments for the nonmovement-related symptoms of PD, such as sleep disturbances and emotional problems, is less strong.
The disease is named after English doctor James Parkinson, who published the first detailed description in An Essay on the Shaking Palsy, in 1817. Public awareness campaigns include World Parkinson's Day (on 11 April, the birthday of James Parkinson) and the use of a red tulip as the symbol of the disease. People with PD who have increased the public's awareness of the condition include boxer Muhammad Ali, comedian Billy Connolly, actor Michael J. Fox, Olympic cyclist Davis Phinney, and actor Alan Alda.
Classification
Parkinson's disease is the most common form of parkinsonism and is also called idiopathic parkinsonism, meaning that it has no identifiable cause. The accumulation of a misfolded protein alpha-synuclein in the brain, and its spread throughout the brain makes Parkinson's disease a neurodegenerative disease classed as a synucleinopathy, and more specifically as an alpha-synucleinopathy (αsynucleinopathy).
Other Parkinson-plus syndromes can have similar movement symptoms but have a variety of associated symptoms. Some of these are also synucleinopathies. Lewy body dementia involves motor symptoms with early onset of cognitive dysfunction and hallucinations which precede motor symptoms. Alternatively, multiple systems atrophy or MSA usually has early onset of autonomic dysfunction (such as orthostasis), and may have autonomic predominance, cerebellar symptom predominance, or Parkinsonian predominance.
Other Parkinson-plus syndromes involve tau, rather than alpha-synuclein. These include progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS). PSP predominantly involves rigidity, early falls, bulbar symptoms, and vertical gaze restriction; it can be associated with frontotemporal dementia symptoms. CBS involves asymmetric parkinsonism, dystonia, alien limb, and myoclonic jerking. These presentation timelines and associated symptoms can help differentiate these similar movement disorders from idiopathic Parkinson disease.
Signs and symptoms
The most recognizable symptoms are movement (motor) related, and include tremor, bradykinesia, rigidity, and shuffling/stooped gait. Non-motor symptoms, including autonomic dysfunction (dysautonomia), neuropsychiatric problems (mood, cognition, behavior or thought alterations), and sensory (especially altered sense of smell) and sleep difficulties may be present as well. Patients may have nonmotor symptoms that precede the onset of motor symptoms including constipation, anosmia, and REM Behavior Disorder. Generally, symptoms such as dementia, psychosis, orthostasis, and more severe falls occur later. Dysphagia can begin at any time during the course of Parkinson's, and affects more than 80% of patients.
Motor
Four motor symptoms are considered as cardinal signs in PD: tremor, slowness of movement (bradykinesia), rigidity, and postural instability.
The most common presenting sign is a coarse, slow tremor of the hand at rest, which disappears during voluntary movement of the affected arm and in the deeper stages of sleep. It typically appears in only one hand, eventually affecting both hands as the disease progresses. Frequency of PD tremor is between 4–6 hertz (cycles per second). A common characteristic of tremor is pill-rolling, the tendency of the index finger and thumb to touch and perform together with a circular movement. The term derives from the similarity between the movement of people with PD and the early pharmaceutical technique of manually making pills.
Bradykinesia is due to disturbances in motor planning of movement initiation, and associated with difficulties along the whole course of the movement process, from planning to initiation to execution of a movement. Performance of sequential and simultaneous movement is impaired. Bradykinesia is the most handicapping symptom of Parkinson's disease, presenting as difficulties with everyday tasks such as dressing, feeding, and bathing. It leads to particular difficulty in carrying out two independent motor activities at the same time, and can be made worse by emotional stress or concurrent illnesses. Paradoxically, people with PD can ride a bicycle or climb stairs more easily than walk on the level. Although most physicians may readily notice bradykinesia, formal assessment requires persons to do repetitive movements with their fingers and feet.
In parkinsonism, rigidity or hypokinesia can be uniform, known as lead-pipe rigidity, or ratcheted, known as cogwheel rigidity. The combination of tremor and increased tone is considered to be at the origin of cogwheel rigidity. Rigidity may be associated with joint pain; such pain being a frequent initial manifestation of the disease. In early stages of PD, rigidity is asymmetrical and tends to affect the neck and shoulder muscles before the muscles of the face and extremities. With the progression of the disease, rigidity typically affects the whole body and reduces the ability to move.
Postural instability is typical in the later stages of the disease, leading to impaired balance and frequent falls, and secondarily to bone fractures, loss of confidence, and reduced mobility. Instability is absent in the initial stages, especially in younger people, especially before the development of bilateral symptoms. Up to 40% of people diagnosed with PD may experience falls, and around 10% may have falls weekly, with the number of falls being related to the severity of PD.
Other recognized motor signs and symptoms include gait and posture disturbances such as festination (rapid shuffling steps and a forward-flexed posture when walking with no flexed arm swing). Other common signs include freezing of gait (brief arrests when the feet seem to get stuck to the floor, especially on turning or changing direction), a slurred, monotonous, quiet voice, mask-like facial expression, and handwriting that gets smaller and smaller.
Cognitive
PD causes neuropsychiatric disturbances ranging from mild to severe including disorders of cognition, mood, behavior, and thought. Cognitive disturbances can occur in the early stages or before diagnosis, and increase in prevalence with duration of the disease. The most common cognitive deficit is executive dysfunction, which can include problems with planning, cognitive flexibility, abstract thinking, rule acquisition, inhibiting inappropriate actions, initiating appropriate actions, working memory, and control of attention. Other cognitive difficulties include slowed cognitive processing speed, impaired recall, and impaired perception and estimation of time. Nevertheless, improvement appears when recall is aided by cues. Visuospatial difficulties are a part of the disease, seen for example when the individual is asked to perform tests of facial recognition and perception of the orientation of drawn lines.
A person with PD has two to six times the risk of dementia compared with the general population. Up to 78% of people with PD have Parkinson's disease dementia. The prevalence of dementia increases with age, and to a lesser degree, duration of the disease. Dementia is associated with a reduced quality of life in people with PD and their caregivers, increased mortality, and a higher probability of needing nursing home care.
Psychosis
Psychosis can be considered a symptom with a prevalence at its widest range from 26 to 83%.Hallucinations or delusions occur in about 50% of people with PD over the course of the illness, and may herald the emergence of dementia. These range from minor hallucinations – sense of passage (something quickly passing beside the person) or sense of presence (the perception of something or someone standing to the side or behind the person) – to full blown vivid, formed visual hallucinations and paranoid ideation. Auditory hallucinations are uncommon in PD, and are rarely described as voices. Psychosis is believed to be an integral part of the disease. A psychosis with delusions and associated delirium is a recognized complication of anti-Parkinson drug treatment. Urinary tract infections (frequent in the elderly) and underlying brain pathology or changes in neurotransmitters or their receptors (e.g., acetylcholine, serotonin) are thought to play a role in psychosis in PD.
Neuropsychiatric
Behavior and mood alterations are more common in PD without cognitive impairment than in the general population and are usually present in PD with dementia. The most frequent mood difficulties are depression, apathy, and anxiety. Depression impacts an estimated 20% to 35% of patients, and may appear at any stage of the disease. It can manifest with symptoms common to the disease process (fatigue, insomnia, and difficulty with concentration), which makes diagnosis difficult. The imbalance and changes in dopamine, serotonin, and noradrenergic hormones and functional imparment are causes of depression in PD-affected people.Suicidal ideation is higher than in the general population, but suicidal attempts themselves are lower. Risk factors for depression include disease onset under age 50, being female, previous history of depression, or severe motor symptoms.
Anxiety has been estimated to have a prevalence in PD-affected people usually around 30–40% and up to 60% has been found. Anxiety with PD is complex and consists of symptoms specific to PD. Anxiety can be higher during motor "off" periods (times when medication is ineffective) and is likely to be diagnosed after diagnosis due to dysfunction of neurotransmitter pathways. PD-affected people experience panic attacks more frequently compared with the general population. Both anxiety and depression have been found to be associated with decreased quality of life. Symptoms can range from mild and episodic to chronic with potential causes being abnormal gamma-aminobutyric acid levels and embarrassment or fear about symptoms or disease. Risk factors for anxiety in PD are disease onset under age 50, women, and off periods. No standard treatment for PD-associated anxiety exists.
Apathy and anhedonia can be defined as a loss of motivation and an impaired ability to experience pleasure and are symptoms classically associated with depression, but differ in PD-affected people in treatment and mechanism. Apathy presents in around 16.5–40%. Symptoms of apathy include reduced initiative/interests in new activities or the world around them, emotional indifference, and loss of affection or concern for others. Apathy is associated with deficits in cognitive functions including executive and verbal memory. Anhedonia occurs in 5–75% of people with PD, depending on the study population assessed and overlap with apathy.
Impulse-control disorders, including pathological gambling, compulsive sexual behavior, binge eating, compulsive shopping, and reckless generosity, can be medication-related, particularly orally active dopamine agonists. The dopamine dysregulation syndrome – with wanting of medication contributing to overuse – is a rare complication of levodopa use.
Punding, complicated, repetitive, aimless, stereotyped behaviors, is another side effect of anti-Parkinson medication.
Gastrointestinal
Gastrointestinal issues in Parkinson's disease include constipation, impaired stomach emptying (gastric dysmotility), and excessive production of saliva can be severe enough to cause discomfort or endanger health. Other upper gastrointestinal symptoms include swallowing impairment (Oropharyngeal dysphagia) and small intestinal bacterial overgrowth.
Individuals with Parkinson's have alpha-synuclein deposits in the digestive tract as well as the brain. Constipation is one of the symptoms associated with an increased risk of PD and may precede diagnosis of PD.
Other
Sleep disorders occur with PD and can be worsened by medications. Symptoms can manifest as daytime drowsiness (including sudden sleep attacks resembling narcolepsy), disturbances in Rapid eye movement sleep, or insomnia.REM behavior disorder may begin years before the development of motor or cognitive elements of PD or dementia with Lewy bodies.
Alterations in the autonomic nervous system can lead to orthostatic hypotension (low blood pressure on standing), oily skin, excessive sweating, urinary incontinence, and altered sexual function.
Changes in perception may include an impaired sense of smell, disturbed vision, pain, and paresthesia (tingling and numbness). These symptoms can occur years before diagnosis of the disease.
Causes
None of the proposed risk factors have been conclusively proven. The most frequently replicated relationships are an increased risk in those exposed to pesticides and a reduced risk in smokers. A possible link exists between PD and Helicobacter pylori infection that can prevent the absorption of some drugs, including levodopa.
Genetic
Research indicates that PD results from a complex interaction between genetic and environmental factors. Around 15% of diagnosed individuals have a first-degree relative who has the disease, and 5–10% have a mutation in genes. Harboring one of these gene mutations may not lead to the disease; susceptibility factors put them at an increased risk, in combination with other factors, which affect age of onset, severity and progression. At least 11 autosomal dominant and nine autosomal recessive gene mutations have been implicated in the development of PD. The autosomal dominant genes include SNCA, PARK3, UCHL1, LRRK2, GIGYF2, HTRA2, EIF4G1, TMEM230, CHCHD2, RIC3, and VPS35. Autosomal recessive genes include PRKN, PINK1, PARK7, ATP13A2, PLA2G6, FBXO7, DNAJC6, SYNJ1, and VPS13C. Some genes are X-linked or have unknown inheritance pattern; those include PARK10, PARK12, and PARK16. A 22q11 deletion is known to be associated with PD. An autosomal dominant form has been associated with mutations in the LRP10 gene.
About 5% of people with PD have mutations in the GBA1 gene. These mutations are present in fewer than 1% of the unaffected population. The risk of developing PD is increased 20–30-fold if these mutations are present. PD associated with these mutations has the same clinical presentation, but an earlier age of onset and a more rapid cognitive and motor decline. This gene encodes glucocerebrosidase. Low levels of this enzyme cause Gaucher's disease.
Alpha-synuclein, a protein encoded by SNCA gene mutations, is the main component of the Lewy bodies that accumulate in the brains of people with PD. Alpha-synuclein activates ataxia telangiectasia mutated, a major DNA damage-repair signaling kinase. In addition, alpha-synuclein activates the non-homologous end joining DNA repair pathway. The aggregation of alpha-synuclein in Lewy bodies appears to be a link between reduced DNA repair and brain-cell death in PD.
Mutations in some genes, including SNCA, LRRK2, and GBA, have been found to be risk factors for sporadic (nonfamilial) PD. Mutations in the gene LRRK2 are the most common known cause of familial and sporadic PD, accounting for around 5% of individuals with a family history of the disease and 3% of sporadic PD. A mutation in GBA presents the greatest genetic risk of developing Parkinsons disease.
Parkinson-related genes are involved in the function of lysosomes, organelles that digest cellular waste products. Lysosomal disorders that reduce the ability of cells to break down alpha-synuclein may cause PD.
Non-genetic
Exposure to pesticides and head injury have each been linked with PD, but the risks are low. Not drinking caffeinated beverages is associated with small increases in risk. Some toxins can cause parkinsonism, including manganese and carbon disulfide.
Medical drugs are implicated in parkinsonism. Drug-induced parkinsonism is normally reversible by stopping the offending agent, such as phenothiazines (chlorpromazine, promazine, etc.); butyrophenones (haloperidol, benperidol, etc.); metoclopramide and Tetrabenazine. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a drug known for causing irreversible parkinsonism that is commonly used in animal-model research.
Low concentrations of urate in the blood are associated with an increased risk.
Other identifiable causes include infections and metabolic derangement. Neurodegenerative disorders may present with parkinsonism, and are also referred to as atypical parkinsonism or parkinson plus syndromes (illnesses with parkinsonism plus some other symptoms distinguishing them from PD) including multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy bodies. Dementia with Lewy bodies is another synucleinopathy and it has close pathological similarities with PD, especially with the subset of PD with dementia known as Parkinson's disease dementia. The relationship between PD and DLB is complex and incompletely understood. It represents a continuum with variable distinguishing clinical and pathological attributes, or it may prove to be separate diseases.
Vascular parkinsonism is the phenomenon of the presence of Parkinson's disease symptoms combined with findings of vascular events (such as a cerebral stroke). The damaging of the dopaminergic pathways is similar in cause for both vascular parkinsonism and idiopathic PD, and so present with similar symptoms. Differentiation can be made with careful bedside examination, history evaluation, and imaging.
Pathophysiology
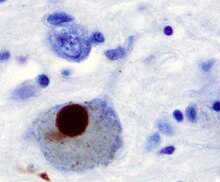
The main pathological characteristics of PD are cell death in the brain's basal ganglia (affecting up to 70% of the dopamine-secreting neurons in the substantia nigra pars compacta by the end of life). In Parkinson's disease, alpha-synuclein becomes misfolded and clump together with other alpha-synuclein. Cells are unable to remove these clumps, and the alpha-synuclein becomes cytotoxic, damaging the cells. These clumps can be seen in neurons under a microscope and are called Lewy bodies. Loss of neurons is accompanied by the death of astrocytes (star-shaped glial cells) and an increase in the number of microglia (another type of glial cell) in the substantia nigra. Severity of progression of the parts of the brain affected by PD can be measured with Braak staging. According to this staging, PD starts in the medulla and the olfactory bulb before moving to the substantia nigra pars compacta and the rest of the midbrain/basal forebrain. Movement symptom onset is associated when the disease begins to affect the substantia nigra pars compacta.
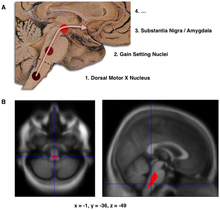
- Schematic initial progression of Lewy body deposits in the first stages of PD, as proposed by Braak and colleagues
- Localization of the area of significant brain volume reduction in initial PD compared with a group of participants without the disease in a neuroimaging study, which concluded that brainstem damage may be the first identifiable stage of PD neuropathology
Five major pathways in the brain connect other brain areas to the basal ganglia. These are known as the motor, oculomotor, associative, limbic, and orbitofrontal circuits. Names indicate the main projection area of each circuit. All are affected in PD, and their disruption causes movement-, attention- and learning-related symptoms of the disease. Scientifically, the motor circuit has been examined the most intensively.
Since 1980, a particular conceptual model of the motor circuit and its alteration with PD has been of influence although some limitations have been pointed out which have led to modifications. In this model, the basal ganglia normally exert a constant inhibitory influence on a wide range of motor systems, preventing them from becoming active at inappropriate times. When a decision is made to perform a particular action, inhibition is reduced for the required motor system, thereby releasing it for activation. Dopamine acts to facilitate this release of inhibition, so high levels of dopamine function tend to promote motor activity, while low levels of dopamine function, such as occur in PD, demand greater exertions of effort for any given movement. The end result of dopamine depletion is to produce hypokinesia, an overall reduction in motor output. Drugs that are used to treat PD, conversely, may produce excessive dopamine activity, allowing motor systems to be activated at inappropriate times and thereby producing dyskinesias.
Brain cell death
One mechanism causing brain cell death results from abnormal accumulation of the protein alpha-synuclein bound to ubiquitin in damaged cells. This insoluble protein accumulates inside neurons forming inclusions, known as Lewy bodies. These bodies first appear in the olfactory bulb, medulla oblongata and pontine tegmentum; individuals at this stage may be asymptomatic or have early nonmotor symptoms (such as loss of sense of smell or some sleep or automatic dysfunction). As the disease progresses, Lewy bodies develop in the substantia nigra, areas of the midbrain and basal forebrain, and finally, the neocortex. These brain sites are the main places of neuronal degeneration in PD, but Lewy bodies may be protective from cell death (with the abnormal protein sequestered or walled off). Other forms of alpha-synuclein (eg oligomers) that are not aggregated into Lewy bodies and Lewy neurites, may in fact be the toxic forms of the protein. In people with dementia, a generalized presence of Lewy bodies is common in cortical areas. Neurofibrillary tangles and senile plaques, characteristic of Alzheimer's disease, are uncommon unless the person has dementia.
Other mechanisms include proteasomal and lysosomal systems dysfunction and reduced mitochondrial activity. Iron accumulation in the substantia nigra is typically observed in conjunction with the protein inclusions. It may be related to oxidative stress, protein aggregation, and neuronal death, but the mechanisms are obscure.
The neuroimmune connection
The neuroimmune interaction is heavily implicated in PD pathology. PD and autoimmune disorders share genetic variations and molecular pathways. Some autoimmune diseases may even increase one's risk of developing PD, up to 33% in one study. Autoimmune diseases linked to protein expression profiles of monocytes and CD4+ T cells are linked to PD. Herpes virus infections can trigger autoimmune reactions to alpha-synuclein, perhaps through molecular mimicry of viral proteins. Alpha-synuclein, and its aggregate form, Lewy bodies, can bind to microglia. Microglia can proliferate and be over-activated by alpha-synuclein binding to MHC receptors on inflammasomes, bringing about a release of proinflammatory cytokines like IL-1β, IFNγ, and TNFα. Activated microglia influence the activation of astrocytes, converting their neuroprotective phenotype to a neurotoxic one. Astrocytes in healthy brains serve to protect neuronal connections. In PD patients, astrocytes cannot protect the dopaminergic connections in the striatum. Microglia present antigens via MHC-I and MHC-II to T cells. CD4+ T cells, activated by this process, are able to cross the blood brain barrier (BBB) and release more proinflammatory cytokines, like interferon-γ (IFNγ), TNFα, and IL-1β. Mast cell degranulation and subsequent proinflammatory cytokine release is implicated in BBB breakdown in PD. Another immune cell implicated in PD are peripheral monocytes and have been found in the substantia nigra of PD patients. These monocytes can lead to more dopaminergic connection breakdown. In addition, monocytes isolated from PD patients express higher levels of the PD-associated protein, LRRK2, compared with non-PD individuals via vasodilation. In addition, high levels of pro-inflammatory cytokines, such as IL-6, can lead to the production of C-reactive protein by the liver, another protein commonly found in PD patients, that can lead to an increase in peripheral inflammation. Peripheral inflammation can affect the gut-brain axis, an area of the body highly implicated in PD. PD patients have altered gut microbiota and colon problems years before motor issues arise. Alpha-synuclein is produced in the gut and may migrate via the vagus nerve to the brainstem, and then to the substantia nigra. Furthermore, the bacteria Proteus mirabilis has been associated with higher levels of alpha-synuclein and an increase of motor symptoms in PD patients. Further elucidation of the causal role of alpha-synuclein, the role of inflammation, the gut-brain axis, as well as an understanding of the individual differences in immune stress responses is needed to better understand the pathological development of PD.
Diagnosis
Physician's initial assessment is typically based on medical history and neurological examination. They access motor symptoms (bradykinesia, rest tremors, etc) using clinical diagnostic criteria. The finding of Lewy bodies in the midbrain on autopsy is usually considered final proof that the person had PD. The clinical course of the illness over time may diverge from PD, requiring that presentation is periodically reviewed to confirm the accuracy of the diagnosis.
Multiple causes can occur for parkinsonism or diseases that look similar. Stroke, certain medications, and toxins can cause "secondary parkinsonism" and need to be assessed during visit. Parkinson-plus syndromes, such as progressive supranuclear palsy and multiple system atrophy, must be considered and ruled out appropriately to begin a different treatment and disease progression (anti-Parkinson's medications are typically less effective at controlling symptoms in Parkinson-plus syndromes). Faster progression rates, early cognitive dysfunction or postural instability, minimal tremor, or symmetry at onset may indicate a Parkinson-plus disease rather than PD itself.
Medical organizations have created diagnostic criteria to ease and standardize the diagnostic process, especially in the early stages of the disease. The most widely known criteria come from the UK Queen Square Brain Bank for Neurological Disorders and the U.S. National Institute of Neurological Disorders and Stroke. The Queen Square Brain Bank criteria require slowness of movement (bradykinesia) plus either rigidity, resting tremor, or postural instability. Other possible causes of these symptoms need to be ruled out. Finally, three or more of the following supportive symptoms are required during onset or evolution: unilateral onset, tremor at rest, progression in time, asymmetry of motor symptoms, response to levodopa for at least five years, the clinical course of at least ten years and appearance of dyskinesias induced by the intake of excessive levodopa. Assessment of sudomotor function through electrochemical skin conductance can be helpful in diagnosing dysautonomia.
When PD diagnoses are checked by autopsy, movement disorders experts are found on average to be 79.6% accurate at initial assessment and 83.9% accurate after refining diagnoses at follow-up examinations. When clinical diagnoses performed mainly by nonexperts are checked by autopsy, the average accuracy is 73.8%. Overall, 80.6% of PD diagnoses are accurate, and 82.7% of diagnoses using the Brain Bank criteria are accurate.
Imaging
Computed tomography (CT) scans of people with PD usually appear normal. Magnetic resonance imaging has become more accurate in diagnosis of the disease over time, specifically through iron-sensitive T2* and susceptibility weighted imaging sequences at a magnetic field strength of at least 3T, both of which can demonstrate absence of the characteristic 'swallow tail' imaging pattern in the dorsolateral substantia nigra. In a meta-analysis, absence of this pattern was highly sensitive and specific for the disease. A meta-analysis found that neuromelanin-MRI can discriminate individuals with Parkinson's from healthy subjects.Diffusion MRI has shown potential in distinguishing between PD and Parkinson-plus syndromes, as well as between PD motor subtypes, though its diagnostic value is still under investigation. CT and MRI are used to rule out other diseases that can be secondary causes of parkinsonism, most commonly encephalitis and chronic ischemic insults, as well as less-frequent entities such as basal ganglia tumors and hydrocephalus.
The metabolic activity of dopamine transporters in the basal ganglia can be directly measured with positron emission tomography and single-photon emission computed tomography scans. It has shown high agreement with clinical diagnoses of PD. Reduced dopamine-related activity in the basal ganglia can help exclude drug-induced Parkinsonism. This finding is nonspecific and can be seen with both PD and Parkinson-plus disorders. In the United States, DaTSCANs are only FDA approved to distinguish PD or Parkinsonian syndromes from essential tremor.
Iodine-123-meta-iodobenzylguanidine myocardial scintigraphy can help locate denervation of the muscles of the heart which can support a PD diagnosis.
Differential diagnosis
Secondary parkinsonism – The multiple causes of parkinsonism can be differentiated through careful history, physical examination, and appropriate imaging. This topic is further discussed in the causes section here.
Parkinson-plus syndrome – Multiple diseases can be considered part of the Parkinson's plus group, including corticobasal syndrome, multiple system atrophy, progressive supranuclear palsy, and dementia with Lewy bodies. Differential diagnosis can be narrowed down with careful history and physical exam (especially focused on the sequential onset of specific symptoms), progression of the disease, and response to treatment. Some key symptoms:
- Corticobasal syndrome – levodopa-resistance, myoclonus, dystonia, corticosensory loss, apraxia, and non-fluent aphasia
- Dementia with Lewy bodies – levodopa resistance, cognitive predominance before motor symptoms, and fluctuating cognitive symptoms, (visual hallucinations are common in this disease)
- Essential tremor – This can at first look like parkinsonism, but has key differentiators. In essential tremor, the tremor gets worse with action (improves in PD), a lack of other symptoms is common in PD, and normal DatSCAN is seen.
- Multiple system atrophy – levodopa resistance, rapidly progressive, autonomic failure, stridor, present Babinski sign, cerebellar ataxia, and specific MRI findings
- Progressive supranuclear palsy – levodopa resistance, restrictive vertical gaze, specific MRI findings, and early and different postural difficulties
Other conditions that can have similar presentations to PD include:
- Arthritis
- Creutzfeldt–Jakob disease
- Depression
- Dystonia
- Fragile X-associated tremor/ataxia syndrome
- Frontotemporal dementia and parkinsonism linked to chromosome 17
- Huntington's disease
- Idiopathic basal ganglia calcification
- Neurodegeneration with brain iron accumulation
- Normal-pressure hydrocephalus
- Obsessional slowness
- Psychogenic parkinsonism
- Wilson's disease
Prevention
Exercise in middle age may reduce the risk of PD later in life.Caffeine appears protective with a greater decrease in risk occurring with a larger intake of caffeinated beverages such as coffee.
Antioxidants, such as vitamins C and E, have been proposed to protect against the disease, but results of studies have been contradictory and no positive effect has been shown. The results regarding fat and fatty acids have been contradictory. Use of nonsteroidal anti-inflammatory drugs (NSAIDs) and calcium channel blockers may be protective. A 2010 meta-analysis found that NSAIDs (apart from aspirin), have been associated with at least a 15% (higher in long-term and regular users) reduction in the incidence of the development of PD. As of 2019 meta-analyses have failed to confirm this link. Multiple studies have demonstrated a link between the use of ibuprofen and a decreased risk of Parkinson's development.
Management
No cure for Parkinson's disease is known. Medications, surgery, and physical treatment may provide relief, improve the quality of a person's life, and are much more effective than treatments for other neurological disorders such as Alzheimer's disease, motor neuron disease, and Parkinson-plus syndromes. The main families of drugs useful for treating motor symptoms are levodopa always combined with a dopa decarboxylase inhibitor and with a COMT inhibitor, dopamine agonists, and MAO-B inhibitors. The stage of the disease and the age at disease onset determine which group is most useful.
Braak staging of PD uses six stages that can identify early, middle, and late stages. The initial stage in which some disability has already developed and requires pharmacological treatment is followed by later stages associated with the development of complications related to levodopa usage, and a third stage when symptoms unrelated to dopamine deficiency or levodopa treatment may predominate.
Treatment in the first stage aims for an optimal trade-off between symptom control and treatment side effects. The start of levodopa treatment may be postponed by initially using other medications, such as MAO-B inhibitors and dopamine agonists, instead, in the hope of delaying the onset of complications due to levodopa use. Levodopa is still the most effective treatment for the motor symptoms of PD and treatment should be prompt in people when their quality of life is impaired. Levodopa-related dyskinesias correlate more strongly with duration and severity of the disease than duration of levodopa treatment.
In later stages, the aim is to reduce PD symptoms, while controlling fluctuations in the effect of the medication. Sudden withdrawals from medication or its overuse must be managed. When oral medications are inadequate in controlling symptoms, surgery (deep brain stimulation or high-intensity focused ultrasound), subcutaneous waking-day apomorphine infusion, and enteral dopa pumps may be useful. Late-stage PD presents challenges requiring a variety of treatments, including those for psychiatric symptoms particularly depression, orthostatic hypotension, bladder dysfunction, and erectile dysfunction. In the final stages of the disease, palliative care is provided to improve a person's quality of life.
A 2020 Cochrane review found no certain evidence that cognitive training is beneficial for people with Parkinson's disease, dementia or mild cognitive impairment. The findings are based on low certainty evidence of seven studies.
Medications
Levodopa
Levodopa is usually the first drug of choice when treating Parkinson's disease and has been the most widely used PD treatment since the 1980s. The motor symptoms of PD are the result of reduced dopamine production in the brain's basal ganglia. Dopamine fails to cross the blood–brain barrier so it cannot be taken as a medicine to boost the brain's depleted levels of dopamine. A precursor of dopamine, levodopa, can pass through to the brain where it is readily converted to dopamine. Administration of levodopa temporarily diminishes the motor symptoms of PD.
Only 5–10% of levodopa crosses the blood–brain barrier. Much of the remainder is metabolized to dopamine elsewhere in the body, causing a variety of side effects, including nausea, vomiting, and orthostatic hypotension.Carbidopa and benserazide are dopa decarboxylase inhibitors that fails to cross the blood–brain barrier and inhibit the conversion of levodopa to dopamine outside the brain, reducing side effects and improving the availability of levodopa for passage into the brain. One of these drugs is usually taken along with levodopa and is availible combined with levodopa in the same pill.
Prlonged use of levodopa is associated with the development of complications, such as involuntary movements (dyskinesias) and fluctuations in the impact of the medication. When fluctuations occur, a person can cycle through phases with good response to medication and reduced PD symptoms (on state), and phases with poor response to medication and increased PD symptoms (off state). Using lower doses of levodopa may reduce the risk and severity of these levodopa-induced complications. A former strategy, called "drug holidays", to reduce levodopa-related dyskinesia and fluctuations was to withdraw levodopa medication for some time which can bring on dangerous side effects such as neuroleptic malignant syndrome and is discouraged. Most people with PD eventually need levodopa and later develop levodopa-induced fluctuations and dyskinesias. Adverse effects of levodopa, including dyskinesias, mistakenly influence patients and providers to delay treatment which reduces potential for optimal results.
Levodopa by itself is available in oral (tablets and capsules), oral inhalation, and infusion form. Inhaled levodopa can be used when oral levodopa therapy has reached a point where "off" periods have increased in length.
COMT inhibitors
During the course of PD, affected people can experience a wearing-off phenomenon, where a recurrence of symptoms occurs after a dose of levodopa, but right before their next dose. Catechol-O-methyltransferase (COMT) is a protein that degrades levodopa before it can cross the blood–brain barrier and these inhibitors allow for more levodopa to cross. They are normally used in the management of later symptoms, but can be used in conjunction with levodopa/carbidopa when a person is experiencing the wearing off-phenomenon with their motor symptoms.
Three COMT inhibitors are used to treat adults with PD and end-of-dose motor fluctuations – opicapone, entacapone, and tolcapone. Tolcapone has been available for but its usefulness is limited by possible liver damage complications requiring liver-function monitoring. Entacapone and opicapone cause little alteration to liver function. Licensed preparations of entacapone contain entacapone alone or in combination with carbidopa and levodopa. Opicapone is a once-daily COMT inhibitor.
Dopamine agonists
Dopamine agonists that bind to dopamine receptors in the brain have similar effects to levodopa. These were initially used as a complementary therapy to levodopa for individuals experiencing levodopa complications (on-off fluctuations and dyskinesias); they are mainly used on their own as first therapy for the motor symptoms of PD with the aim of delaying the initiation of levodopa therapy, thus delaying the onset of levodopa's complications. Dopamine agonists include bromocriptine, pergolide, pramipexole, ropinirole, piribedil, cabergoline, apomorphine, and lisuride.
Though dopamine agonists are less effective than levodopa at controlling PD motor symptoms, they are effective enough to manage these symptoms in the first years of treatment. Dyskinesias due to dopamine agonists are rare in younger people who have PD, but along with other complications, become more common with older age at onset. Thus, dopamine agonists are the preferred initial treatment for younger-onset PD, and levodopa is preferred for older-onset PD.
Dopamine agonists produce side effects, including drowsiness, hallucinations, insomnia, nausea, and constipation. Side effects appear with minimal clinically effective doses giving the physician reason to search for a different drug. Agonists have been related to impulse-control disorders (such as increased sexual activity, eating, gambling, and shopping) more strongly than other antiparkinson medications. They tend to be more expensive than levodopa.
Apomorphine, a dopamine agonist, may be used to reduce off periods and dyskinesia in late PD. It is administered only by intermittent injections or continuous subcutaneous infusions. Secondary effects such as confusion and hallucinations are common, individuals receiving apomorphine treatment should be closely monitored. Two dopamine agonists administered through skin patches (lisuride and rotigotine) are useful for people in the initial stages and possibly to control off states in those in advanced states. Due to an increased risk of cardiac fibrosis with ergot-derived dopamine agonists (bromocriptine, cabergoline, dihydroergocryptine, lisuride, and pergolide), they should only be considered for adjunct therapy to levodopa.
MAO-B inhibitors
MAO-B inhibitors (safinamide, selegiline and rasagiline) increase the amount of dopamine in the basal ganglia by inhibiting the activity of monoamine oxidase B, an enzyme that breaks down dopamine. They have been found to help alleviate motor symptoms when used as monotherapy (on their own); when used in conjunction with levodopa, time spent in the off phase is reduced. Selegiline has been shown to delay the need for beginning levodopa, suggesting that it might be neuroprotective and slow the progression of the disease. An initial study indicated that selegiline in combination with levodopa increased the risk of death, but this has been refuted.
Common side effects are nausea, dizziness, insomnia, sleepiness, and (in selegiline and rasagiline) orthostatic hypotension. MAO-Bs are known to increase serotonin and cause a potentially dangerous condition known as serotonin syndrome.
Other drugs
Other drugs such as amantadine may be useful as treatment of motor symptoms, but evidence for use is lacking. Anticholinergics should not be used for dyskinesia or motor fluctuations but may be considered topically for drooling. A diverse range of symptoms beyond those related to motor function can be treated pharmaceutically. Examples are the use of quetiapine or clozapine for psychosis, cholinesterase inhibitors or memantine for dementia, and modafinil for excessive daytime sleepiness. In 2016, pimavanserin was approved for the management of PD psychosis. Doxepin and rasagline may reduce physical fatigue in PD.
Surgery

Treating motor symptoms with surgery was once a common practice but the discovery of levodopa has decreased the amount of procedures. Studies have led to great improvements in surgical techniques, so surgery can be used in people with advanced PD for who drug therapy is no longer sufficient. Surgery for PD can be divided in two main groups – lesional and deep brain stimulation (DBS). Target areas for DBS or lesions include the thalamus, globus pallidus, or subthalamic nucleus. DBS involves the implantation of a medical device called a neurostimulator, which sends electrical impulses to specific parts of the brain. DBS is recommended for people who have PD with motor fluctuations and tremor inadequately controlled by medication, or to those who are intolerant to medication lacking severe neuropsychiatric problems. Other less common surgical therapies involve intentional formation of lesions to suppress overactivity of specific subcortical areas. For example, pallidotomy involves surgical destruction of the globus pallidus to control dyskinesia.
Four areas of the brain have been treated with neural stimulators in PD. These are the globus pallidus interna, thalamus, subthalamic nucleus, and pedunculopontine nucleus. DBS of the globus pallidus interna improves motor function, while DBS of the thalamic DBS improves tremor, but has little impact on bradykinesia or rigidity. DBS of the subthalamic nucleus is usually avoided if a history of depression or neurocognitive impairment is present. DBS of the subthalamic nucleus is associated with a reduction in medication. Pedunculopontine nucleus DBS remains experimental at present. Generally, DBS is associated with 30–60% improvement in motor score evaluations.
Rehabilitation
Exercise programs are recommended in people with PD. Some evidence shows that speech or mobility problems can improve with rehabilitation, although studies are scarce and of low quality. Regular physical exercise with or without physical therapy can be beneficial to maintain and improve mobility, flexibility, strength, gait speed, and quality of life. When an exercise program is performed under the supervision of a physiotherapist, more improvements occur in motor symptoms, mental and emotional functions, daily living activities, and quality of life compared with a self-supervised exercise program at home. Clinical exercises may be an effective intervention targeting overall well-being of individuals with Parkinson's. Improvement in motor function and depression may happen.
In improving flexibility and range of motion for people experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation techniques. As for gait and addressing the challenges associated with the disease such as hypokinesia, shuffling, and decreased arm swing, physiotherapists have a variety of strategies to improve functional mobility and safety. Areas of interest concerning gait during rehabilitation programs focus on improving gait speed, the base of support, stride length, and trunk and arm-swing movement. Strategies include using assistive equipment (pole walking and treadmill walking), verbal cueing (manual, visual, and auditory), exercises (marching and PNF patterns), and altering environments (surfaces, inputs, open vs. closed). Strengthening exercises have shown improvements in strength and motor function for people with primary muscular weakness and weakness related to inactivity with mild to moderate PD, but reports show an interaction between strength and the time the medications were taken. Therefore, people with PD should perform exercises 45 minutes to one hour after medications when they are capable. Deep diaphragmatic breathing exercises are beneficial in improving chest-wall mobility and vital capacity decreased by a forward flexed posture and respiratory dysfunctions in advanced PD. Exercise may improve constipation. If exercise reduces physical fatigue in PD remains unclear.
Strength training exercise has been shown to increase manual dexterity in PD patients after exercising with manual putty. This positively affects everyday life when gripping for PD patients.
The Lee Silverman voice treatment (LSVT) is one of the most widely practiced treatments for speech disorders associated with PD. Speech therapy and specifically LSVT may improve speech.Occupational therapy (OT) aims to promote health and quality of life by helping people with the disease to participate in a large percentage of their daily living activities. Few studies have been conducted on the effectiveness of OT, and their quality is poor, although with some indication that it may improve motor skills and quality of life for the duration of the therapy.
Palliative care
The goal of Palliative care is to improve quality of life for both the patient and family by providing relief from the symptoms and stress of illnesses. As Parkinson's is uncurable, treatments focus on slowing decline and improving quality of life and are therefore palliative.
Palliative care should be involved earlier, rather than later, in the disease course. Palliative care specialists can help with physical symptoms, emotional factors such as loss of function and jobs, depression, fear, and existential concerns.
Along with offering emotional support to both the affected person and family, palliative care addresses goals of care. People with PD may have difficult decisions to make as the disease progresses, such as wishes for feeding tube, noninvasive ventilator or tracheostomy, wishes for or against cardiopulmonary resuscitation, and when to use hospice care. Palliative-care team members can help answer questions and guide people with PD on these complex and emotional topics to help them make decisions based on values.
Muscles and nerves that control the digestive process may be affected by PD, resulting in constipation and gastroparesis (prolonged emptying of stomach contents). A balanced diet, based on periodical nutritional assessments, is recommended, and should be designed to avoid weight loss or gain and minimize the consequences of gastrointestinal dysfunction. As the disease advances, swallowing difficulties (dysphagia) may appear. Using thickening agents for liquid intake and an upright posture when eating may be useful; both measures reduce the risk of choking. Gastrostomy can be used to deliver food directly into the stomach.
Levodopa and proteins use the same transportation system in the intestine and the blood–brain barrier, thereby competing for access. Taking them together results in reduced effectiveness of the drug. Therefore, when levodopa is introduced, excessive protein consumption is discouraged in favour of a well-balanced Mediterranean diet. In advanced stages, additional intake of low-protein products such as bread or pasta is recommended for similar reasons. To minimize interaction with proteins, levodopa should be taken 30 minutes before meals. At the same time, regimens for PD restrict proteins during breakfast and lunch, allowing protein intake in the evening.
Prognosis

|
no data
< 5
5–12.5
12.5–20
20–27.5
27.5–35
35–42.5 |
42.5–50
50–57.5
57.5–65
65–72.5
72.5–80
> 80 |
PD invariably progresses with time. A severity rating method known as the Unified Parkinson's disease rating scale (UPDRS) is the most commonly used metric for a clinical study. A modified version known as the MDS-UPDRS is also used. An older scaling method known as the Hoehn and Yahr scale (originally published in 1967), and a similar scale known as the Modified Hoehn and Yahr scale, have been used. The Hoehn and Yahr scale defines five basic stages of progression.
Motor symptoms may advance aggressively in the early stages of the disease and more slowly later. Untreated, individuals are expected to lose independent ambulation after an average of eight years and be bedridden after 10 years. Medication has improved the prognosis of motor symptoms. In people taking levodopa, the progression time of symptoms to a stage of high dependency from caregivers may be over 15 years. Predicting what course the disease will take for a given individual is difficult. Age is an appropriate predictor of disease progression. The rate of motor decline is greater in those with less impairment at the time of diagnosis, while cognitive impairment is more frequent in those who are over 70 years of age at symptom onset.
Disability is mainly related to nonmotor symptoms of the disease and therapies exist to improve these. Nevertheless, the relationship between disease progression and disability is independent of each other. Disability is initially related to motor symptoms. As the disease advances, disability is more related to motor symptoms that are uncontrollable by medication, such as swallowing and speech difficulties, and gait and balance problems; and to levodopa-induced complications, which appear in up to 50% of individuals after five years of levodopa usage. Finally, after ten years most people with the disease have autonomic disturbances, sleep problems, mood alterations and cognitive decline. These symptoms, especially cognitive decline, greatly increase disability.
The life expectancy of people with PD is reduced.Mortality ratios are around twice those of unaffected people. Cognitive decline and dementia, old age at onset, a more advanced disease state, and presence of swallowing problems are all mortality risk factors. A disease pattern mainly characterized by tremor as opposed to rigidity, though, predicts an improved survival. Death from aspiration pneumonia is twice as common in individuals with PD as in the healthy population.
In 2016, PD resulted in about 211,000 deaths globally, an increase of 161% since 1990. The overall death rate increased by 19% to 1.81 per 100,000 people during that time.
Epidemiology
PD is the second most common neurodegenerative disorder after Alzheimer's disease and affects approximately seven million people globally and one million people in the United States. The proportion in a population at a given time is about 0.3% in industrialized countries. PD is more common in the elderly and rates rise from 1% in those over 60 years of age to 4% of the population over 80. The mean age of onset is around 60 years, although 5–10% begin between the ages of 20 and 50 is classified as young onset PD. Males are affected at a ratio of around 3:2 compared with females. PD may be less prevalent in those of African and Asian ancestry, although this finding is disputed. The number of new diagnoses per year of PD is between 8–18 per 100,000 person–years.
The age-adjusted rate of Parkinson's disease in Estonia is 28.0/100,000 person–years. The Estonian rate has been stable between 2000 and 2019. The incidence of Parkinson's disease has increased in China. It is estimated that China will have nearly half of the Parkinson's disease population in the world in 2030. By 2040 the number of patients is expected to grow to approximately 14 million people; this growth has been referred to as the Parkinson's pandemic.
History

Early sources, including an Egyptian papyrus, an Ayurvedic medical treatise, the Bible, and Galen's writings, describe symptoms resembling those of PD. After Galen, no references unambiguously related to PD appear until the 17th century. In the 17th and 18th centuries, Franciscus Sylvius, Hieronymus David Gaubius, John Hunter and Auguste François Chomel wrote about elements of the disease.
In 1817, James Parkinson published his essay reporting six people with paralysis agitans.An Essay on the Shaking Palsy described the characteristic resting tremor, abnormal posture and gait, paralysis and diminished muscle strength, and the way that the disease progresses over time. Early neurologists who made further additions to the knowledge of the disease include Trousseau, Gowers, Kinnier Wilson and Erb, and Jean-Martin Charcot, whose studies between 1868 and 1881 increased the understanding of the disease. Among other advances, he made the distinction between rigidity, weakness and bradykinesia. He championed the renaming of the disease in honor of James Parkinson.
In 1912, Frederic Lewy described microscopic particles in affected brains, later named Lewy bodies. In 1919, Konstantin Tretiakoff reported that the substantia nigra was the main cerebral structure affected, but this finding was rejected until it was confirmed by further studies published by Rolf Hassler in 1938. The underlying biochemical changes in the brain were identified in the 1950s, due largely to the work of Arvid Carlsson on the neurotransmitter dopamine and Oleh Hornykiewicz on its role on PD. In 1997, alpha-synuclein was found to be the main component of Lewy bodies by Spillantini, Trojanowski, Goedert and others.
Anticholinergics and surgery (lesioning of the corticospinal pathway or some of the basal ganglia structures) were the only treatments until the arrival of levodopa, which reduced their use dramatically.Levodopa was first synthesized in 1911 by Casimir Funk, but it received little attention until the mid 20th century. It entered clinical practice in 1967 and brought about a revolution in the management of PD. By the late 1980s deep brain stimulation introduced by Alim Louis Benabid and colleagues at Grenoble, France, emerged as a possible treatment.
Society and culture
Cost
The costs of PD to society are high, but methodological issues in research and differences between countries make precise calculations difficult. The largest share of direct cost comes from inpatient care and nursing homes, while the share coming from medication is substantially lower. Indirect costs are high, due to reduced productivity and the burden on caregivers. In addition to economic costs, PD reduces quality of life of those with the disease and their caregivers.
A study based on 2017 data estimated the US economic PD burden at $51.9 billion, including direct medical costs of $25.4 billion and $26.5 billion in indirect and non-medical costs. The Medicare program bears the largest share of medical costs, as most PD patients are over age 65. The projected total economic burden surpasses $79 billion by 2037. These findings highlight the need for interventions to reduce PD incidence, delay disease progression, and alleviate symptom burden that may reduce the future economic burden of PD.
Advocacy
The birthday of James Parkinson, 11 April, has been designated as World Parkinson's Day. A red tulip was chosen by international organizations as the symbol of the disease in 2005; it represents the 'James Parkinson' tulip cultivar, registered in 1981 by a Dutch horticulturalist. Advocacy organizations include the National Parkinson Foundation, which has provided more than $180 million in care, research, and support services since 1982,Parkinson's Disease Foundation, which has distributed more than $115 million for research and nearly $50 million for education and advocacy programs since its founding in 1957 by William Black; the American Parkinson Disease Association, founded in 1961; and the European Parkinson's Disease Association, founded in 1992.
Notable cases

Actor Michael J. Fox was 29 when diagnosed with PD, and has used his diagnosis to increase awareness of the disease. Fox embraced his Parkinson's in television roles, sometimes appearing without medication to further illustrate the effects of the condition. He has written four autobiographies in which his fight against the disease plays a major role, and appeared before the United States Congress without medication to illustrate the effects of the disease.The Michael J. Fox Foundation aims to develop a cure for Parkinson's disease. Fox received an honorary doctorate in medicine from Karolinska Institutet for his contributions to research in Parkinson's disease.
Professional cyclist and Olympic medalist Davis Phinney, who was diagnosed with young-onset Parkinson's at age 40, started the Davis Phinney Foundation in 2004 to support PD research, focusing on quality of life for people with the disease.
Boxer Muhammad Ali showed signs of PD when he was 38, but was undiagnosed until he was 42, and has been called the "world's most famous Parkinson's patient". Whether he had PD or parkinsonism related to boxing is unresolved.
At the time of his suicide in 2014, Robin Williams, the American actor and comedian, had been diagnosed with PD. According to his widow, his autopsy found diffuse Lewy body disease, while the autopsy used the term diffuse Lewy body dementia. Dennis Dickson, a spokesperson for the Lewy Body Dementia Association, clarified the distinction by stating that diffuse Lewy body dementia is more commonly called diffuse Lewy body disease and refers to the underlying disease process.Ian G. McKeith, professor and researcher of Lewy body dementias, commented that Williams' symptoms and autopsy findings were explained by dementia with Lewy bodies.
Research
As of 2022, no disease-modifying drugs (drugs that target the causes or damage) are approved for Parkinson's, so this is a major focus of Parkinson's research. Active research directions include the search for new animal models of the disease and studies of the potential usefulness of gene therapy, stem cell transplants, and neuroprotective agents. To aid in earlier diagnosis, research criteria for identifying prodromal biomarkers of the disease have been established.
The role of the gut–brain axis and the gut flora in PD are recognized but the mechanism that causes gastrointestinal symptoms is unclear.
Gene therapy
Gene therapy typically involves the use of a noninfectious virus (i.e., a viral vector such as the adeno-associated virus) to shuttle genetic material into a part of the brain. Approaches have involved the expression of growth factors to prevent damage (Neurturin – a GDNF-family growth factor), and enzymes such as glutamic acid decarboxylase (GAD – the enzyme that produces GABA), tyrosine hydroxylase (the enzyme that produces L-DOPA) and catechol-O-methyl transferase (COMT – the enzyme that converts L-DOPA to dopamine). No safety concerns have been reported but the approaches have largely failed in phase two clinical trials. The delivery of GAD showed promise in phase two trials in 2011, but while effective at improving motor function, was inferior to DBS. Follow-up studies in the same cohort have suggested persistent improvement.
Neuroprotective treatments
A vaccine that primes the human immune system to destroy alpha-synuclein, PD01A (developed by Austrian company, Affiris), entered clinical trials and a phase one report in 2020 suggested safety and tolerability. In 2018, an antibody, PRX002/RG7935, showed preliminary safety evidence in stage I trials supporting continuation to stage II trials.
Cell-based therapies
Beginning in the early 1980s, fetal, porcine, carotid or retinal tissues have been used in cell transplants, in which dissociated cells are injected into the substantia nigra in the hope that they will incorporate themselves into the brain in a way that replaces the dopamine-producing cells that have been lost. These sources of tissues have been largely replaced by induced pluripotent stem cell derived dopaminergic neurons, as this is thought to represent a more feasible source of tissue. Initial evidence showed mesencephalic dopamine-producing cell transplants being beneficial, but long-term benefit is undetermined. An additional problem was the excess release of dopamine by the transplanted tissue resulting in dyskinesia. In 2020, a first in human clinical trial reported the transplantation of induced pluripotent stem cells into the brain of a person with PD.
Pharmaceutical
Ventures have been undertaken to explore antagonists of adenosine receptors (specifically A2A) as an avenue for novel drugs for Parkinson's. Of these, istradefylline has emerged as the most successful medication and was approved for medical use in the United States in 2019. It is approved as an add-on treatment to the levodopa/carbidopa regime.
External links
| Classification | |
|---|---|
| External resources |
|
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
| |||||||||||||||||||||
|
Diseases of the nervous system, primarily CNS
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation |
|
||||||||||||||||||||||||
|
Brain/ encephalopathy |
|
||||||||||||||||||||||||
| Both/either |
|
||||||||||||||||||||||||
| Dopaminergics |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anticholinergics | |||||||||||
| Others | |||||||||||
| |||||||||||
| National | |
|---|---|
| Other | |