
Ralfinamide
Подписчиков: 0, рейтинг: 0
Not to be confused with Rufinamide.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.120.272 |
| Chemical and physical data | |
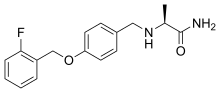
| Formula | C17H19FN2O2 |
| Molar mass | 302.349 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ralfinamide (INN) (code names NW-1029, FCE-26742A, PNU-0154339E) is a multimodal drug which is under investigation by Newron Pharmaceuticals for the treatment of neuropathic pain and other pain conditions such as post-operative dental pain.
It has a relatively complex pharmacology, acting as a mixed voltage-gated sodium channel blocker (including Nav1.7),N-type calcium channel blocker,noncompetitive NMDA receptor antagonist, and monoamine oxidase B inhibitor.
It has thus far progressed as far as phase IIb/phase III clinical trials. In 2010 it failed a phase II trial for lower back pain. Encouraging Phase II results have been announced for neuropathic pain.
See also
- List of investigational analgesics
- Safinamide, different fluorine position
- Evenamide, structurally-related antipsychotic in development
- Lacosamide, used for partial-onset seizures and diabetic neuropathic pain
- Ziconotide, FDA approved peptide for chronic neuropathic pain
External links
| Monoaminergics |
|
|---|---|
| Ion channel blockers |
|
| Others |
|
| Calcium |
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium |
|
||||||||||||||||||||||||
| Sodium |
|
||||||||||||||||||||||||
| Chloride |
|
||||||||||||||||||||||||
| Others |
|
||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||