
Talampanel
Подписчиков: 0, рейтинг: 0
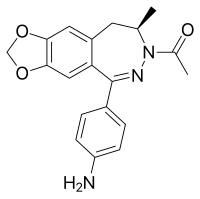 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.001 |
| Chemical and physical data | |
| Formula | C19H19N3O3 |
| Molar mass | 337.379 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Talampanel (INN; development codes GYKI 537773 and LY300164) is a drug which has been investigated for the treatment of epilepsy,malignant gliomas, and amyotrophic lateral sclerosis (ALS).
As of May 2010, results from the trial for ALS have been found negative. Talampanel is not currently under development.
Talampanel acts as a non-competitive antagonist of the AMPA receptor, a type of ionotropic glutamate receptor in the central nervous system.
It showed effectiveness for epilepsy in clinical trials but its development was suspended due to its poor pharmacokinetic profile, namely a short terminal half-life (3 hours) that necessitated multiple doses per day.