
Triprolidine
Triprolidine
Подписчиков: 0, рейтинг: 0
 | |
| Clinical data | |
|---|---|
| Trade names | Actidil, Myidil, Actifed (in the latter combined with pseudoephedrine and either dextromethorphan or guaifenesin) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 4% oral |
| Protein binding | 90% |
| Metabolism | Hepatic (CYP2D6) |
| Elimination half-life | 4–6 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.934 |
| Chemical and physical data | |
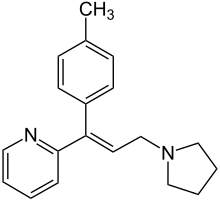
| Formula | C19H22N2 |
| Molar mass | 278.399 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 60 °C (140 °F) |
| Solubility in water | 500 mg/mL (20 °C) |
| |
| |
|
| |
Triprolidine is an over-the-counter antihistamine with anticholinergic properties. It is used to combat the symptoms associated with allergies and is sometimes combined with other cold medications designed to provide general relief for flu-like symptoms. As with many antihistamines, the most common side effect is drowsiness.
It was patented in 1948 and came into medical use in 1953.
See also
| Benzimidazoles | |
|---|---|
| Diarylmethanes |
|
| Ethylenediamines | |
| Tricyclics | |
| Others |
|
| For topical use | |
| H1 |
|
||||
|---|---|---|---|---|---|
| H2 |
|
||||
| H3 |
|
||||
| H4 |
|
||||
| mAChRs |
|
||||
|---|---|---|---|---|---|
|
Precursors (and prodrugs) |
|||||