
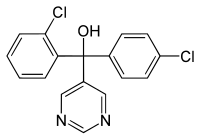
Fenarimol
Подписчиков: 0, рейтинг: 0
| |
| Names | |
|---|---|
|
IUPAC name
(R/S)-2,4′-Dichloro-α-(pyrimidin-5-yl)benzhydryl alcohol
| |
| Other names
α-(2-Chlorophenyl)-α-(4-chlorophenyl)-5-pyrimidinemethanol
| |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.056.432 |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H12Cl2N2O | |
| Molar mass | 331.20 g·mol−1 |
| Appearance | Colorless powder with aromatic odor |
| Melting point | 117 to 119 °C (243 to 246 °F; 390 to 392 K) |
| Boiling point | 240 °C (464 °F; 513 K) (decomposition) |
| 13.7 mg/L at 25 °C | |
| Solubility in other solvents | Soluble in acetone, xylene and methanol |
| Vapor pressure | 65 μ Pa (25 °C) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
>2000 mg·kg−1 (oral, Rat) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fenarimol, sold under the tradenames Bloc, Rimidin and Rubigan, is a fungicide which acts against rusts, blackspot and mildew fungi. It is used on ornamental plants, trees, lawns, tomatoes, peppers, eggplants, cucumbers and melons. It is mainly used to control powdery mildew. It works by inhibiting the fungus's biosynthesis of important steroid molecules (via blockade of the CYP51 enzyme).
History
Fenarimol was developed by Eli Lilly & Company around 1971.
As of early 2018, derivatives of this compound are being researched in an open source manner for possible treatment of eumycetoma.
Synthesis
Fenarimol is made by the reaction of 2,4'-dichlorobenzophenone with an organolithium pyrimidine made via bromine-lithium exchange.
External links
- Fenarimol in the Pesticide Properties DataBase (PPDB)
| Phytoestrogens |
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mycoestrogens |
|
||||||||||||||||||||||||
| Synthetic | |||||||||||||||||||||||||
| Metalloestrogens | |||||||||||||||||||||||||
| ER |
|
||||||
|---|---|---|---|---|---|---|---|
| GPER |
|
||||||
