
Amastatin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.532 |
| Chemical and physical data | |
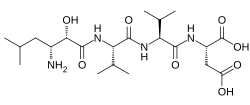
| Formula | C21H38N4O8 |
| Molar mass | 474.555 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3. It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase (aminopeptidase M/N), bacterial leucyl aminopeptidase (Aeromonas proteolytica aminopeptidase), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and, to a lesser extent, glutamyl aminopeptidase (aminopeptidase A), as well as other aminopeptidases. It does not inhibit arginyl aminopeptidase (aminopeptidase B). Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin in vivo. It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides.