
DU-41165
 | |
| Clinical data | |
|---|---|
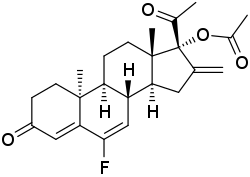
| Other names | 6-Fluoro-16-methylene-17α-acetoxy-δ6-retroprogesterone; 6-Fluoro-16-methylene-17α-hydroxy-9β,10α-pregna-4,6-diene-3,20-dione 17α-acetate; 6-Fluoro-16-methylene-3,20-dioxo-9β,10α-pregna-4,6-dien-17α-yl acetate |
| Routes of administration |
By mouth |
| Drug class | Progestin; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H29FO4 |
| Molar mass | 400.490 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
DU-41165, also known as 6-fluoro-16-methylene-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed. It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone. The drug shows extremely high potency as a progestogen in animals. It has been found to possess 158% of the relative binding affinity of promegestone for the progesterone receptor expressed in rat uterus (relative to 74% for the closely related progestin DU-41164). DU-41165 also showed 28% of the affinity of RU-28362 for the glucocorticoid receptor expressed in rat liver, but no affinity for the mineralocorticoid receptor expressed in rat kidney (<0.003% of that of RU-26752). The drug showed no androgenic, anabolic, or estrogenic activity in animals, but did show some antiandrogenic and glucocorticoid activity at high doses. Although highly potent in animals, DU-41165 produced little or no progestogenic effect at dosages of 50 and 200 µg/day in women, suggesting major species differences. DU-41165 has been studied as a potential photoaffinity label for the progesterone receptor.
| GR |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PR |
|
||||||
|---|---|---|---|---|---|---|---|
|
mPR (PAQR) |
|
||||||