
Methallenestril
 | |
| Clinical data | |
|---|---|
| Trade names | Cur-men, Ercostrol, Geklimon, Novestrine, Vallestril (also spelled Vallestrol or Vallestryl) |
| Other names | Methallenoestril; Methallenestrol; Methallenoestrol; Horeau's acid; Allenestrol 6-methyl ether; α,α-Dimethyl-β-ethylallenolic acid 6-methyl ether; β-Ethyl-6-methoxy-α,α-dimethyl-2-naphthalenepropionic acid |
| Routes of administration |
By mouth |
| Drug class | Nonsteroidal estrogen |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.485 |
| Chemical and physical data | |
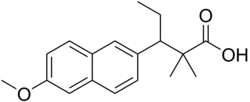
| Formula | C18H22O3 |
| Molar mass | 286.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Methallenestril (INN) (brand names Cur-men, Ercostrol, Geklimon, Novestrine, Vallestril), also known as methallenoestril (BAN) and as methallenestrol, as well as Horeau's acid, is a synthetic nonsteroidal estrogen and a derivative of allenolic acid and allenestrol (specifically, a methyl ether of it) that was formerly used to treat menstrual issues but is now no longer marketed. It is a seco-analogue of bisdehydrodoisynolic acid, and although methallenestril is potently estrogenic in rats, in humans it is only weakly so in comparison.Vallestril was a brand of methallenestril issued by G. D. Searle & Company in the 1950s. Methallenestril is taken by mouth. By the oral route, a dose of 25 mg methallenestril is approximately equivalent to 1 mg diethylstilbestrol, 4 mg dienestrol, 20 mg hexestrol, 25 mg estrone, 2.5 mg conjugated estrogens, and 0.05 mg ethinylestradiol.
See also
| ER |
|
||||||
|---|---|---|---|---|---|---|---|
| GPER |
|
||||||