
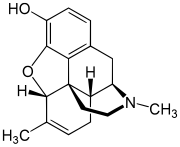
Methyldesorphine
 | |
| Clinical data | |
|---|---|
| Other names | 3-Hydroxy-6,N-dimethyl- 4,5-epoxymorphin-6-en |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.036.474 |
| Chemical and physical data | |
| Formula | C18H21NO2 |
| Molar mass | 283.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
|
| |
Methyldesorphine is an opioid analgesic. First synthesized in Germany in 1940 and patented in the US in 1952, it has a high potential for abuse as with any potent opioid agonist, and is sometimes found along with desomorphine as a component of the home-made opioid mixture known as "Krokodil" used in Russia and the neighboring former Soviet republics. It is approximately 15 times more potent than morphine as an analgesic but if the 6-7 bond is saturated, the β isomer is some 50 times more potent than morphine.
Methyldesorphine is listed as a Schedule I Narcotic controlled substance under the Controlled Substances Act 1970 in the United States with a DEA ACSCN of 9302 and zero annual aggregate manufacturing quota. The free base conversion ratio of the hydrochloride is 0.89.