
Amentoflavone
| |

| |
| Names | |
|---|---|
|
IUPAC name
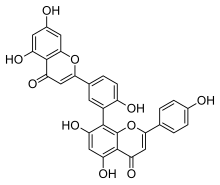
(4′,5,7-Trihydroxyflavone)-(3′→8)-(4′,5,7-trihydroxyflavone)
| |
|
Systematic IUPAC name
8-[5-(5,7-Dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Didemethyl-ginkgetin
3′,8″-Biapigenin | |
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider |
|
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H18O10 | |
| Molar mass | 538.464 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Amentoflavone is a biflavonoid (bis-apigenin coupled at 8 and 3′ positions, or 3′,8″-biapigenin) constituent of a number of plants including Ginkgo biloba, Chamaecyparis obtusa (hinoki), Biophytum sensitivum, Selaginella tamariscina,Hypericum perforatum (St. John's Wort) and Xerophyta plicata.
Amentoflavone can interact with many medications by being a potent inhibitor of CYP3A4 and CYP2C9, which are enzymes responsible for the metabolism of some drugs in the body. It is also an inhibitor of human cathepsin B.
Amentoflavone has a variety of in vitro activities including antimalarial activity, anticancer activity (which may, at least in part, be mediated by its inhibition of fatty acid synthase), and antagonist activity at the κ-opioid receptor (Ke = 490 nmol L−1) as well as activity at the allosteric benzodiazepine site of the GABAA receptor as a negative allosteric modulator.
See also
External links
-
 Media related to Amentoflavone at Wikimedia Commons
Media related to Amentoflavone at Wikimedia Commons
|
Flavones and their conjugates
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
|
||||||||||||
| Glycosides |
|
||||||||||||
| Acetylated | |||||||||||||
| Sulfated glycosides |
Theograndin I and II
|
||||||||||||
| Polymers | |||||||||||||
| Drugs | |||||||||||||
| MOR |
|
|---|---|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted | |
| Others |
|